Covid 19 Pandemic
Covid 19 Pandemic
Long COVID Conditions – Fatigue 03
Section 3
ROLE OF VAGUS NERVE IN INFLAMMATION
There are two methods by which peripheral inflammation may exacerbate CNS inflammation: leaky regions in the blood-brain barrier and the vagus nerve. The vagus nerve, also known as the tenth cranial nerve, is the autonomic nervous system’s longest nerve. To moderate oxygen demand, this nerve has parasympathetic regulation of various organs involved in breathing, including the lungs, heart, and diaphragm, which might contribute to fatigue.
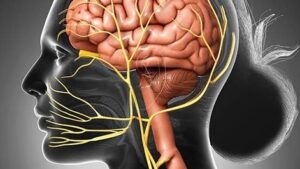
Afferents from the vagal nerve tend to transfer pro-inflammatory responses from cells in the peripheral organs to the CNS. From CNS signals, vagal efferents tend to produce anti-inflammatory responses in peripheral tissue. The vagal afferents transmit pro-inflammatory signals from the peritoneum and organs such as the lung, gut, ear, spleen, liver, and lung to trigger inflammatory cytokines in specific centers in the CNS. From these centers Pro-inflammatory signal from the periphery cause increase pro-inflammatory cytokine production in parts of the brain that impact tiredness and sleep.
In contrast, activation of the vagal efferents caused by cholinergic mechanisms in the brain, such as muscarinic acetylcholine receptors dorsal motor nucleus (DMN), may cause anti-inflammatory effects in peripheral tissues. Vagal efferents originating in the brainstem and extending to peripheral organs, may help to reduce inflammation. Vagotomy, which inhibits the vagus nerve, may reveal directional alterations between the periphery and the CNS. Inhibiting vagal afferents with a vagotomy and administering inflammatory chemicals to the periphery suggest that at low doses, the vagal afferent nerves may be a primary mechanism for translating peripheral inflammation to central inflammation, affecting behavior such as sleep. Vagotomy cannot prevent central inflammation caused by peripheral inflammation as a result of high concentrations of inflammatory stimuli which suggests that the blood-brain barrier is ineffective and also involved in CNS inflammation.
In individuals with rheumatoid arthritis, vagal nerve stimulation is utilized as an alternate treatment to decrease TNF. Vagal nerve stimulation may help those with inflammatory bowel disease. The effects of this anti-inflammatory medication are due in part to the stimulation of cholinergic neurons, which causes peripheral inflammation to be suppressed, which subsequently reduces CNS inflammation. In fact, vagal nerve stimulation decreased daily drowsiness, improved tiredness, and lowered whole blood cell levels of TNF-, IFN-, IL-6, and IL-1 in people with Sjörgen’s syndrome.
NEUROVASCULAR
Cerebral blood flow is a critical component in supplying nutrients and oxygen to cells in the central nervous system The neurovasculature also eliminates waste products like as carbon dioxide and signaling chemicals, as well as providing enough energy reserves. Cellular and performance problems may result from a decline in blood supply in a region around neurons in the CNS. Multiple sclerosis, type 1 diabetes, Alzheimer’s disease, and celiac disease are all linked to hypoperfusion. Neural activity is synchronized with localized cerebral blood flow, which is known as neurovascular coupling. Increases in the production of vasoactive chemicals are linked to increased brain activity, which may affect vascular hemodynamics directly or indirectly.
Sleep, and Autoimmune and Related Disorders Endothelial cells, smooth muscle, astrocytes, neurons, and pericytes make up the neurovascular unit at the level of the cerebral microvasculature, which includes arterioles and capillaries, and it is influenced by microglia and perivascular macrophages. Changes in metabolism and inflammation may also affect astrocyte end-feet, affecting cerebral blood flow (CBF). The neurovascular unit regulates blood flow throughout the brain and is influenced by the surrounding cells’ energy demands as well as the vasoconstrictive and vasodilative substances secreted by these cells, such as catecholamines and dopamine.
Monoamines released by neurons have both vasodilative and vasoconstrictive properties, which can influence blood flow. Pro-inflammatory molecules tend to be vasodilative, reduce vascular resistance, and increase cerebral CBF, whereas monoamines released by neurons have both vasodilative and vasoconstrictive properties, which can influence blood flow. Vasoconstrictive drugs usually raise vascular resistance and lower CBF.
CITATIONS
- Fatigue Survey Results Released • AARDA. Available online at: https://www. aarda.org/fatigue-survey-results-released/ (accessed March 17, 2019).
- Zielinski MR, Systrom DM and Rose NR (2019) Fatigue, Sleep, and Autoimmune and Related Disorders. Front. Immunol. 10:1827. doi: 10.3389/fimmu.2019.01827
- Chalah MA, Ayache SS. Is there a link between inflammation and fatigue in multiple sclerosis? J Inflamm Res. (2018) 11:253–64. doi: 10.2147/JIR.S167199
- Griggs S, Morris NS. Fatigue among adults with type 1 diabetes mellitus and implications for self-management: an integrative review. Diabetes Educ. (2018) 44:325–39. doi: 10.1177/0145721718782148
- Torres J, Mehandru S, Colombel J-F, Peyrin-Biroulet L. Crohn’s disease. Lancet. (2017) 389:1741–55. doi: 10.1016/S0140-6736(16)31711-1
- Nguyen MH, Bryant K, O’Neill SG. Vitamin D in SLE: a role in pathogenesis and fatigue? A review of the literature. Lupus. (2018) 27:2003– 11. doi: 10.1177/0961203318796293
- Hajjar J, Kutac C, Rider NL, Seeborg FO, Scalchunes C, Orange J. Fatigue and the wear-off effect in adult patients with common variable immunodeficiency. Clin Exp Immunol. (2018) 194:327–38. doi: 10.1111/cei.13210
- Hajjar J, Guffey D, Minard CG, Orange JS. Increased incidence of fatigue in patients with primary immunodeficiency disorders: prevalence and associations within the US immunodeficiency network registry. J Clin Immunol. (2017) 37:153–65. doi: 10.1007/s10875-016-0367-1
- Junghaenel DU, Christodoulou C, Lai J-S, Stone AA. Demographic correlates of fatigue in the US general population: results from the patient- reported outcomes measurement information system (PROMIS) initiative. J Psychosom Res. (2011) 71:117–23. doi: 10.1016/j.jpsychores.2011.04.007
- Enns MW, Bernstein CN, Kroeker K, Graff L, Walker JR, Lix LM, et al. The association of fatigue, pain, depression and anxiety with work and activity impairment in immune mediated inflammatory diseases. PLoS ONE. (2018) 13:e0198975. doi: 10.1371/journal.pone.0198975
- Aghaei N, Karbandi S, Gorji MAH, Golkhatmi MB, Alizadeh B. Social support in relation to fatigue symptoms among patients with multiple sclerosis. Indian J Palliat Care. (2016) 22:163–7. doi: 10.4103/0973-1075.179610
- Flachenecker P. Autoimmune diseases and rehabilitation. Autoimmun Rev. (2012) 11:219–25. doi: 10.1016/j.autrev.2011.05.01
- Cook KF, Molton IR, Jensen MP. Fatigue and aging with a disability. Arch Phys Med Rehabil. (2011) 92:1126–33. doi: 10.1016/j.apmr.2011.02.017
- Agarwal N, Kumar V. Burden of lupus on work: issues in the employment of
individuals with lupus. Work. (2016) 55:429–39. doi: 10.3233/WOR-162398
Finsterer J, Mahjoub SZ. Fatigue in healthy and diseased individuals. Am J
Hosp Palliat Care. (2014) 31:562–75. doi: 10.1177/1049909113494748

- Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int. (2016) 36:685–95.doi: 10.1007/s00296-015-3415-x
- Sabes-Figuera R, McCrone P, Hurley M, King M, Donaldson AN, Ridsdale L.
- The hidden cost of chronic fatigue to patients and their families. BMC Healt Serv Res. (2010) 10:56. doi: 10.1186/1472-6963-10-56
- Boissoneault J, Letzen J, Robinson M, Staud R. Cerebral blood flow
and heart rate variability predict fatigue severity in patients with chronic fatigue syndrome. Brain Imaging Behav. (2018) 13:789–97. doi: 10.1007/s11682-018-9897-x
- Vichaya EG, Dantzer R. Inflammation-induced motivational changes: perspective gained by evaluating positive and negative valence systems. CurrOpinBehav Sci. (2018) 22:90–5. doi: 10.1016/j.cobeha.2018.01.008
- Vichaya EG, Hunt SC, Dantzer R. Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice. Neuropsychopharmacology. (2014) 39:2884–90. doi: 10.1038/npp.2014.141 Yang Q, Liu R, Yu Q, Bi Y, Liu G. Metabolic regulation of inflammasomes in inflammation. Immunology. (2019) 157: 95–109. doi: 10.1111/imm.13056 Belarbi K, Cuvelier E, Destée A, Gressier B, Chartier-Harlin M-C. NADPH oxidases in Parkinson’s disease: a systematic review. Mol Neurodegener. (2017) 12:84. doi: 10.1186/s13024-017-0225-5
- Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. (2015) 265:35–52. doi: 10.1111/imr.12286
- Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. (2017) 2:17023. doi: 10.1038/sigtrans.2017.23
- Barker BR, Taxman DJ, Ting JP-Y. Cross-regulation between the IL-1β/IL- 18 processing inflammasome and other inflammatory cytokines. CurrOpin Immunol. (2011) 23:591–7. doi: 10.1016/j.coi.2011.07.005
- Groslambert M, Py BF. Spotlight on the NLRP3 inflammasome pathway. J Inflamm Res. (2018) 11:359–74. doi: 10.2147/JIR.S141220
- Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. (2014) 5:352. doi: 10.3389/fphys.2014.00352
- Zielinski MR, Gerashchenko D, Karpova SA, Konanki V, McCarley RW, Sutterwala FS, et al. The NLRP3 inflammasome modulates sleep and NREM sleep delta power induced by spontaneous wakefulness, sleep deprivation and lipopolysaccharide. Brain Behav Immun. (2017) 62:137–50. doi: 10.1016/j.bbi.2017.01.012
- Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non- phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav Immun. (2017) 61:306–16. doi: 10.1016/j.bbi.2016.12.014
- Lei Y, Chen C-J, Yan X-X, Li Z, Deng X-H. Early-life lipopolysaccharide exposure potentiates forebrain expression of NLRP3 inflammasome proteins and anxiety-like behavior in adolescent rats. Brain Res. (2017) 1671:43–54. doi: 10.1016/j.brainres.2017.06.014
- Wu P-J, Liu H-Y, Huang T-N, Hsueh Y-P. AIM 2 inflammasomes regulate neuronal morphology and influence anxiety and memory in mice. Sci Rep. (2016) 6:32405. doi: 10.1038/srep32405
- Wong M-L, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. (2016) 21:797–805. doi: 10.1038/mp.2016.46
- Zhang Z, Ma X, Xia Z, Chen J, Liu Y, Chen Y, et al. NLRP3 inflammasome activation mediates fatigue-like behaviors in mice via neuroinflammation. Neuroscience. (2017) 358:115–23. doi: 10.1016/j.neuroscience.2017.06.048 McGeough MD, Wree A, Inzaugarat ME, Haimovich A, Johnson CD, Peña CA, et al. TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J Clin Invest. (2017) 127:4488–97. doi: 10.1172/JCI90699
- Kopitar-Jerala N. The role of interferons in inflammation and inflammasome activation. Front Immunol. (2017) 8:873. doi: 10.3389/fimmu.2017.00873 Labzin LI, Lauterbach MAR, Latz E. Interferons and inflammasomes: cooperation and counterregulation in disease. J Allergy Clin Immunol. (2016) 138:37–46. doi: 10.1016/j.jaci.2016.05.010
- Li X, Zhang X, Pan Y, Shi G, Ren J, Fan H, et al. mTOR regulates NLRP3 inflammasome activation via reactive oxygen species in murine lupus. Acta BiochimBiophys Sin. (2018) 50:888–96. doi: 10.1093/abbs/ gmy088
- Cade BE, Chen H, Stilp AM, Louie T, Ancoli-Israel S, Arens R, et al. Associations of variants In the hexokinase 1 and interleukin 18 receptor